0:07-0:17
Multiple sclerosis is a chronic, progressive, neurodegenerative demyelinating disease affecting the
central nervous system, leading to neurological disability.
0:18-0:31
In the USA, more than 900,000 adults have been estimated to be living with Multiple Sclerosis.
Approximately 85 percent of people with MS receive a diagnosis of relapse remitting Multiple Sclerosis.
0:32-0:53
According to the National Multiple Sclerosis Society, Glatiramer Acetate injection is a disease modifying
treatment option with a well-established safety profile for the treatment of relapsing remitting multiple
sclerosis to include clinically isolated syndrome, relapsing remitting disease and active secondary
progressive disease in adults.
0:54-1:07
In a blinded placebo-controlled trial with Glatiramer acetate injection 20 milligrams per milliliter, the
most common adverse reactions were injection site reactions, vasodilatation, rash, dyspnea and chest
pain.
1:08-1:28
Adverse reactions were usually mild in intensity. No new adverse reactions appeared in subjects treated
with Glatiramer Acetate injection 40 milligrams per milliliter three times per week as compared to
subjects treated with Glatiramer Acetate injection 20 milligrams per milliliter per day in clinical trials and
during post marketing experience.
1:29-1:39
In another blinded placebo-controlled trial with Glatiramer Acetate injection 40 milligrams per milliliter
three times per week, the most common adverse reactions were injection site reactions.
1:40-1:54
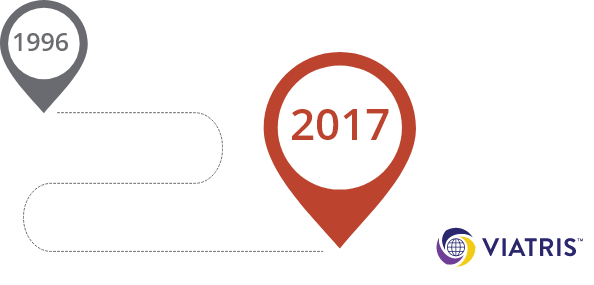
COPAXONE®, reference Glatiramer Acetate injection was approved by the FDA in 1996, with the first
generic Glatiramer Acetate injection receiving approval between 2015 and 2017.
1:55-2:07
How is a generic drug similar to the originator product?
A generic drug should be therapeutically equivalent to its brand name counterpart, which is approved
by the FDA based on extensive tests and procedures.
2:08-2:28
It is important to note that the standards of similarity between a generic product and the reference
drug are no different than that demonstrated between batches of the same brand manufactured. Thus,
generic Glatiramer Acetate injection products meeting the stringent requirements for therapeutic
equivalents were introduced between 2015 and 2017.
2:29-2:38
Of course, science never stands still. At Viatris, our understanding of non-communicable diseases and
how to treat them effectively grows every day.
2:39-2:54
As diseases have become more and more complex, the medications and delivery methods have evolved
to treat those diseases, and so have the FDA's tests and requirements for demonstrating therapeutic
equivalence between generic and branded drugs.
2:56-3:17
In 2017, after a thorough review, the FDA approved VIATRIS' Glatiramer Acetate injection, 20 milligrams
per milliliter and 40 milligrams per milliliter as AP rated or therapeutically equivalent to Teva’s
COPAXONE, 20 milligrams per milliliter and 40 milligrams per milliliter, respectively.
3:19-3:34
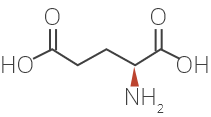
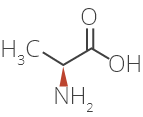
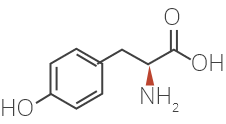
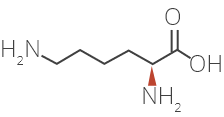
Glatiramer Acetate is a synthetic amino acid copolymer.
Although the mode of action is still not fully understood, it is presumed that Glatiramer Acetate acts in
peripheral circulation by inducing Glatiramer Acetate specific T-cells.
3:35-3:53
Clinical trials and experiments indicate that Glatiramer Acetate exerts its therapeutic activity by
immunomodulating various levels of the immune response, especially by the induction of
immunomodulatory T helper Type 2 clones, thus exerting protective anti-inflammatory action.
3:54-4:13
In the periphery glatiramer acetate binds to major histocompatibility complex class 2 molecules on
antigen presenting cells, resulting in down regulation of pro inflammatory T helper 1 cells and
increase production of anti-inflammatory T helper 2 cells.
4:14-4:30
The Glatiramer Acetate induced T helper two cells, then cross the blood brain barrier and enter the
central nervous system, where they get reactivated in the presence of myelin antigens, resulting in
reduced production of pro inflammatory T helper 1 cells.
4:31-4:50
Glatiramer Acetate peptides compete with myelin peptides such as myelin oligodendrocyte
glycoprotein and proteolipid protein for binding to major histocompatibility complex class 2 molecules
on antigen presenting cells and can preferentially displace them.
4:51-4:59
As a result, differentiation of auto aggressive T helper 1 cells is reduced mediating a shift to T helper
2 and 3 cells.
5:00-5:13
Glatiramer Acetate may also increase the levels of neurotrophic factors such as brain derived
neurotrophic factor and insulin like growth factor, helping in myelination and survival of neurons and
functioning of glial cells.
5:14-5:25
Overall, Glatiramer Acetate leads to increased production of anti-inflammatory cells and has additional
neurotrophic effects resulting in neuroprotection.
5:27-5:42
INDICATION
GLATIRAMER ACETATE INJECTION is indicated for the treatment of patients with relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
5:43-5:53
IMPORTANT SAFETY INFORMATION
Contraindication: GLATIRAMER ACETATE INJECTION is contraindicated in patients with known hypersensitivity to glatiramer acetate or mannitol
5:54-6:56
Immediate Post Injection Reactions: Approximately 16% of patients exposed to GLATIRAMER ACETATE INJECTION 20 mg per mL compared to 4% of those on placebo, and approximately 2% of patients exposed to GLATIRAMER ACETATE INJECTION 40 mg per mL compared to none on placebo experienced a constellation of symptoms that may occur immediately (within seconds to minutes, with the majority of symptoms observed within 1 hour) after injection and included at least 2 of the following: flushing, chest pain, palpitations, tachycardia, anxiety, dyspnea, throat constriction, and urticaria. In general, these symptoms have their onset several months after the initiation of treatment, although they may occur earlier, and a given patient may experience one or several episodes of these symptoms. Typically, the symptoms were transient and self-limited and did not require treatment; however, there have been reports of patients with similar symptoms who received emergency medical care.
6:57-7:45
Chest pain: Transient chest pain (at least one episode) was experienced by 13% of GLATIRAMER ACETATE INJECTION 20 mg per mL patients compared to 6% of placebo patients, and approximately 2% of GLATIRAMER ACETATE INJECTION 40 mg per mL patients compared to 1% on placebo. While some episodes of chest pain occurred in the context of the immediate post-injection reactions that are described, many did not. The temporal relationship of this chest pain to an injection was not always known. The pain was usually transient, often unassociated with other symptoms, and appeared to have no clinical sequelae. Some patients experienced more than one such episode, and episodes usually began at least one month after the initiation of treatment.
7:47-8:23
Lipoatrophy and Skin Necrosis: At injection sites, localized lipoatrophy and, rarely, injection site skin necrosis may occur. Lipoatrophy occurred in approximately 2% of patients exposed to glatiramer acetate injection 20 mg per mL compared to none on placebo, and 0.5% of patients exposed to GLATIRAMER ACETATE INJECTION 40 mg per mL compared to none on placebo. Lipoatrophy may occur at various times after treatment onset (sometimes after several months) and is thought to be permanent. There is no known therapy for lipoatrophy.
8:24-8:52
Potential Effects on Immune Response: Because GLATIRAMER ACETATE INJECTION can modify immune response, it may interfere with immune functions. For example, treatment with GLATIRAMER ACETATE INJECTION may interfere with recognition of foreign antigens in a way that would undermine the body’s tumor surveillance and its defenses against infection. There is no evidence that GLATIRAMER ACETATE INJECTION does this, but there has not been a systematic evaluation of this risk.
8:53-9:15
Hepatic Injury: Cases of hepatic injury, some severe, including liver failure and hepatitis with jaundice, have been reported with GLATIRAMER ACETATE INJECTION. Hepatic injury has occurred from days to years after initiating treatment with GLATIRAMER ACETATE INJECTION. If signs or symptoms of liver dysfunction occur, consider discontinuation of GLATIRAMER ACETATE INJECTION.
9:16-10:00
Glatiramer Acetate Products and Administration Errors: Medication errors have occurred when glatiramer acetate products are administered with incompatible autoinjectors. Not all glatiramer acetate products have a marked optional compatible autoinjector for administration. Using an optional autoinjector that is not compatible for use with Mylan’s GLATIRAMER ACETATE INJECTION may increase the risk for medication errors, such as dose omission or administration of a partial dose. Ensure the device is compatible for use with the specific glatiramer acetate product by referring to the autoinjector labeling. The availability of compatible autoinjectors for each glatiramer acetate product may change with time.
10:01-10:57
Common Adverse Reactions: The most common adverse reactions observed in controlled studies of GLATIRAMER ACETATE INJECTION 20 mg per mL (≥ 10% and ≥ 1.5 times higher than placebo) were injection site reactions (ISRs), vasodilatation, rash, dyspnea, and chest pain. The most common adverse reactions observed in controlled studies of GLATIRAMER ACETATE INJECTION 40 mg per mL (≥ 10% and ≥ 1.5 times higher than placebo) were ISRs. ISRs were one of the most common adverse reactions leading to discontinuation of GLATIRAMER ACETATE INJECTION. ISRs, such as erythema, pain, pruritus, mass, edema, hypersensitivity, fibrosis, and atrophy, occurred at a higher rate with GLATIRAMER ACETATE INJECTION than placebo.